Asahi Kasei microcrystalline cellulose (MCC) products bring a key difference compared with standard MCC products: their high performance stemming from innovative particle morphology.
Why are Asahi Kasei MCCs The Smart Choice for your formulations?
- Facilitating formulations
- Facilitate challenging formulations, solve tableting issues and enable unique and patient-friendly dosage forms.
- The base for quality
- Contribute to the quality improvement of your formulations by less black particles, less impurities and high-quality consistency.
- Production efficiency
- Contribute to your production efficiency by enabling high-speed tableting and reducing production issues.
Products

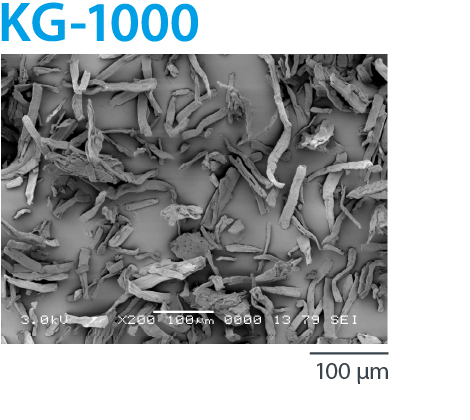
CEOLUS™ KG is a highly compactible MCC with fibrous particles. It enables poorly compactible and/or high dose formulations. It solves tableting issues such as insufficient hardness, sticking or capping.
Functional benefits
- Enables poorly compactible and/or high dose formulations
- Solves tableting issues
Insufficient hardness, sticking, capping, high friability - Enables unique and patient-friendly dosage forms
MUPS, ODT, multiple-layer tablets, multiple-API-combination tablets, small tablets, mini tablets - Enables low-pressure tableting
Applicable for pressure-sensitive API tablets and coated granules

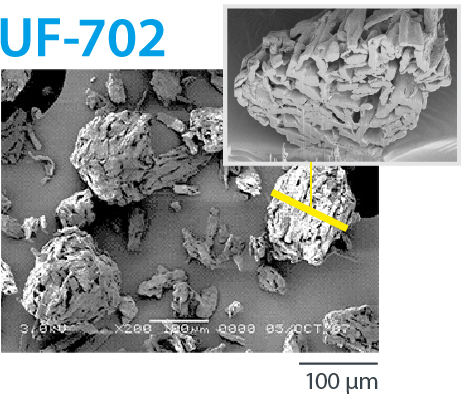
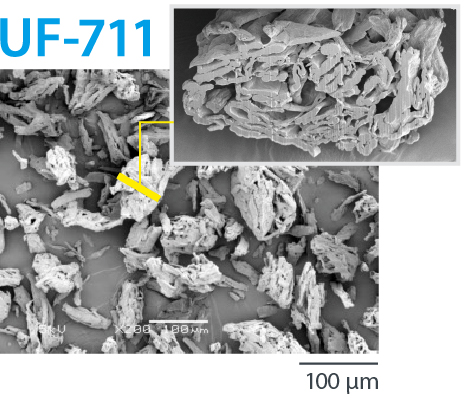
CEOLUS™ UF is a porous MCC with balance of compactibility and flowability. It enables a balance of tablet hardness and tablet disintegration. It solves tableting issues such as insufficient API content uniformity or over-lubrication.
Functional benefits
- Enables a balance of tablet hardness and tablet disintegration
- Solves tableting issues
Insufficient API content uniformity, over-lubrication - Enables poorly flowable and/or low dose formulations
- Enables high speed tableting
Grade lineup
| Grade | Average particle size (μm) |
Bulk density (g/cm³) |
Repose angle (°) |
Loss on drying (%) |
Water absorption (%) |
Oil absorption (%) |
|
|---|---|---|---|---|---|---|---|
 Highly compactible MCC with fibrous particles |
KG-1000 | 50 | 0.12 | 57 | 2.0-6.0 | 290 | 210 |
| KG-802 | 50 | 0.21 | 49 | 2.0-6.0 | 230 | 160 | |
 Porous MCC with balance of compactibility and flowability |
UF-702 | 90 | 0.29 | 34 | 2.0-6.0 | 240 | 160 |
| UF-711 | 50 | 0.22 | 42 | 2.0-6.0 | 240 | 150 |
All values are presented only for the purpose of basic reference and not as specifications.
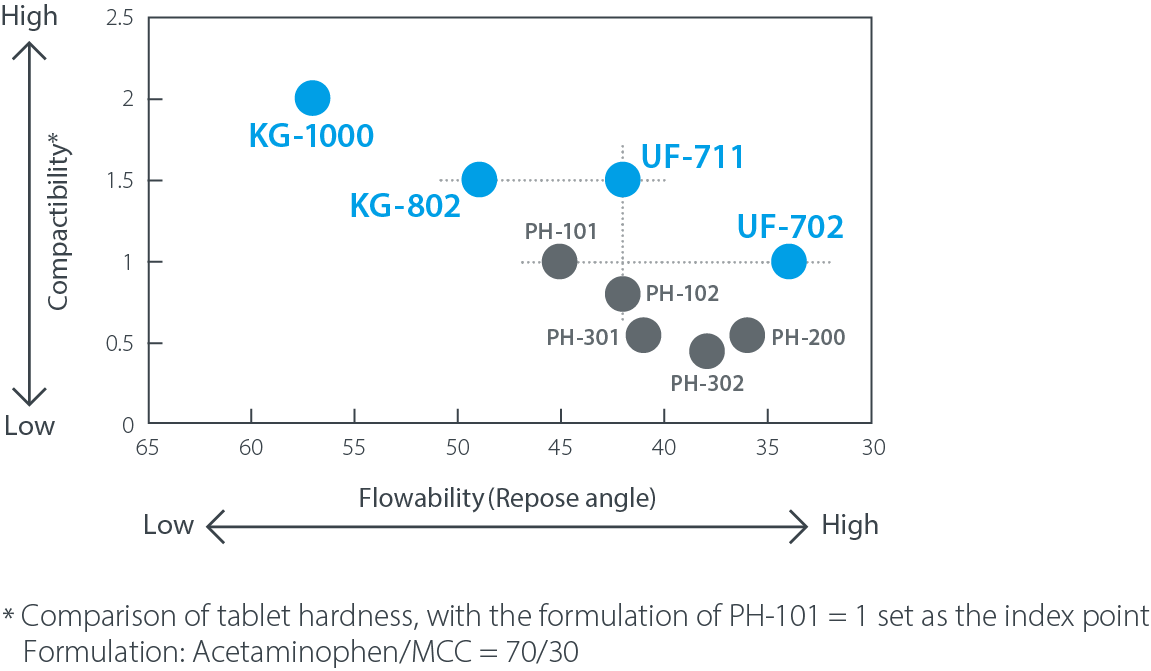
Map of CEOLUS™ Compactibility vs. Flowability

A 100% MCC sphere, CELPHERE™ facilitates precise dissolution profiles of controlled release formulations by its high sphericity and narrow particle size distribution.
Functional benefits
- Facilitates precise dissolution profile of controlled released formulation
- Yield improvement
Reduces agglomeration, tolerance with high stress and coating machine varieties
Grade lineup
General properties
| Grade | Particle size range (μm) | Sphericity | Bulk density (g/cm³) | Water absorption (%) |
|---|---|---|---|---|
| CP-102 | 106-212 | 1.2 | 0.83 | 100 |
| CP-203 | 150-300 | 1.1 | 0.87 | 100 |
| CP-305 | 300-500 | 1.1 | 0.97 | 110 |
| CP-507 | 500-710 | 1.2 | 0.97 | 70 |
| CP-708 | 710-850 | 1.2 | 0.93 | 65 |
All values are presented only for the purpose of basic reference and not as specifications.
Solutions made possible by Asahi Kasei MCC products
Capping prevention
-
- Problem
- Increasing compression force due to poor API compactibility and/or high API dosage leads to higher capping concern.
-
- Solution
- Highly compactible MCC decreases the risk of capping at even high compression force because it strengthens bonding of tablets.
Balance of tablet hardness and tablet disintegration
-
- Problem
- In high shear granulation, it is difficult to achieve the target tablet hardness and tablet disintegration as granulation conditions affect these parameters.
-
- Solution
- Porous MCC with high swellability helps reduce the impact to tablet hardness and tablet disintegration / dissolution by granulation.
Controlled release formulation
-
- Problem
- Granules are often damaged in the coating process and damaged granules affect dissolution.
-
- Solution
- A 100% MCC sphere can reduce impact on dissolution by its mechanical strength.
Asahi Kasei Functional Additives Division
The Asahi Kasei Functional Additives Division offers MCCs for oral solid dosage forms of pharmaceuticals and nutraceuticals.
We have been supplying MCC products in the Japanese pharmaceutical market for over 50 years. Our products now hold a majority share in this market because of their reliability.
A noteworthy advantage of our MCC products is their ability to facilitate challenging and/or innovative formulations of pharmaceuticals and nutraceuticals that customers are bringing to market.
About Asahi Kasei
Asahi Kasei is a diversified Japanese chemical company
with over a century of history.
We operate in the three business sectors of Material,
Homes, and Health Care.
-

-
Material
Environmental Solutions Mobility & Industrial Life Innovation
-

-
Homes
Homes
Construction Materials
-

-
Health Care
Pharmaceuticals Medical Care Acute Critical Care

Nobeoka, Miyazaki, Japan, cradle of Asahi Kasei